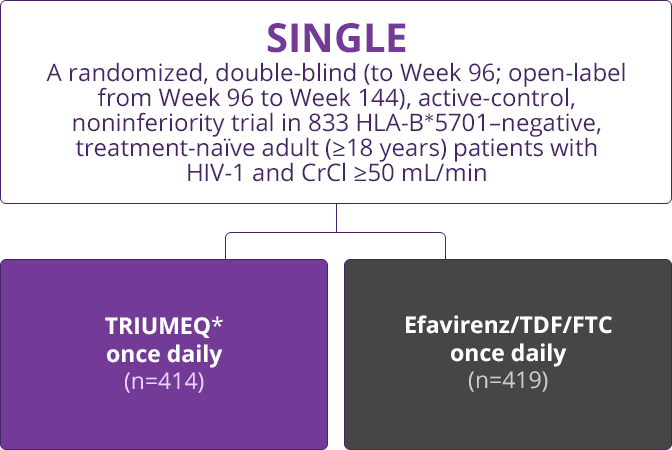
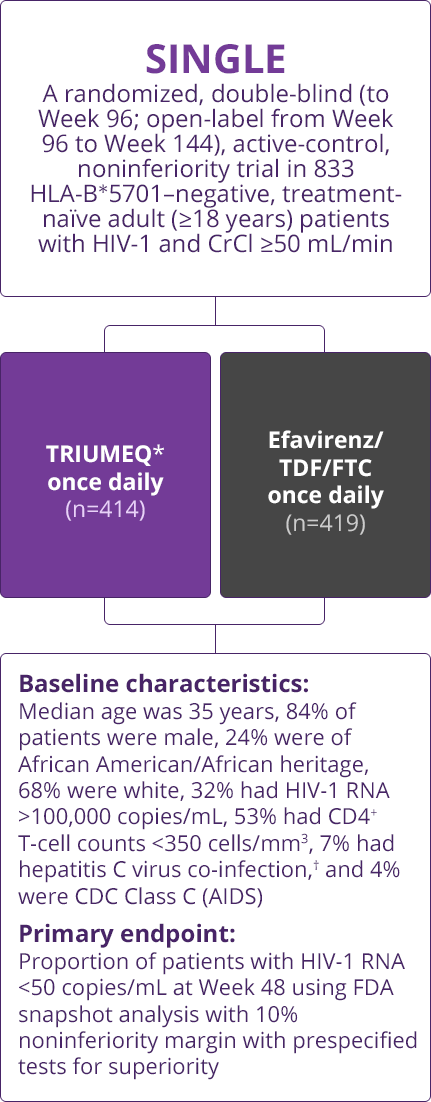
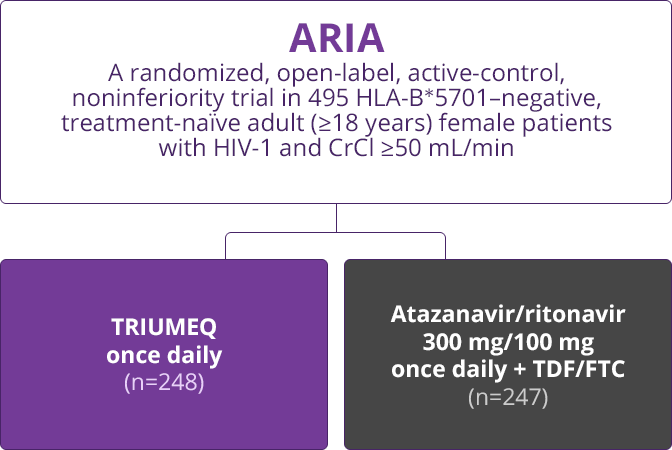
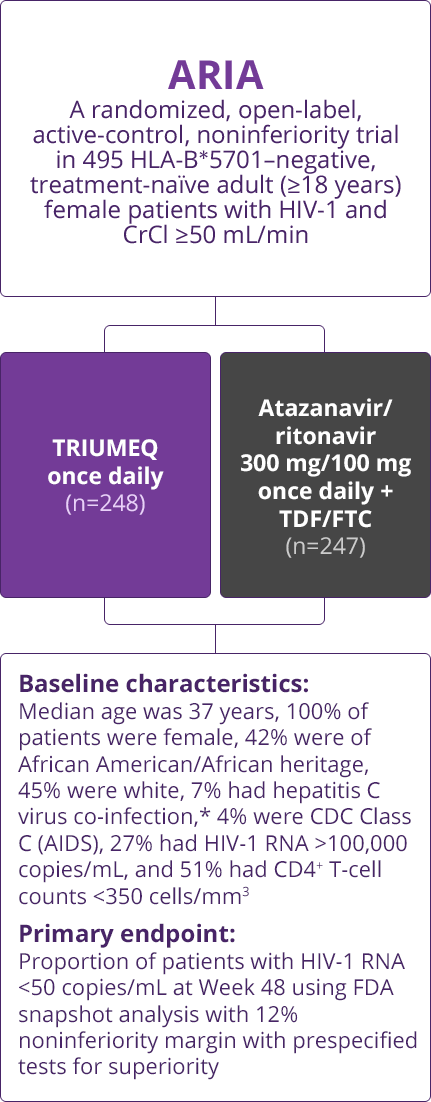
Trial Designs

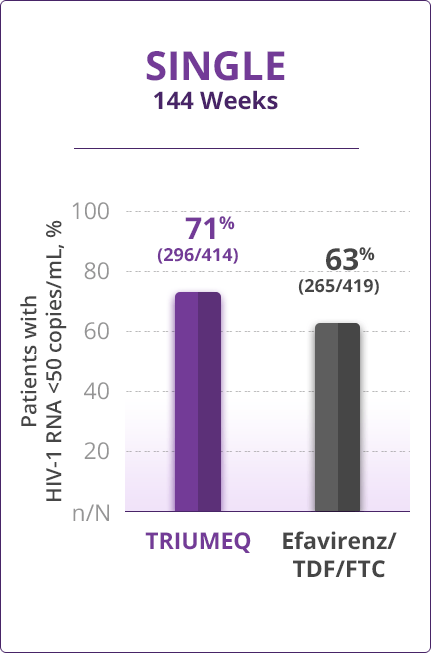
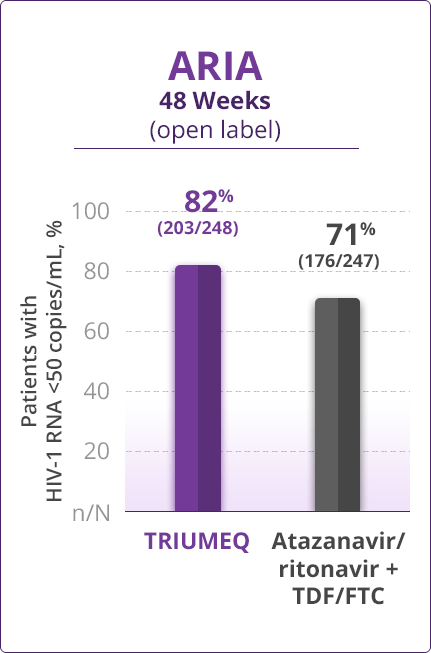
Virologic Response
- Direct comparisons across trials should not be made due to differing trial designs


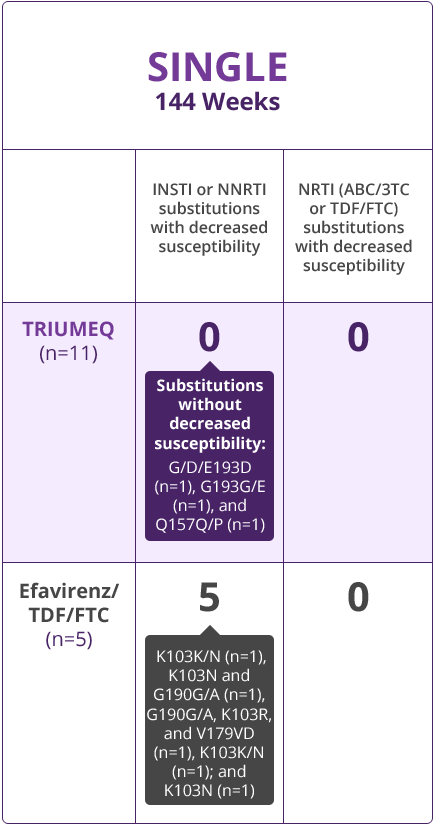
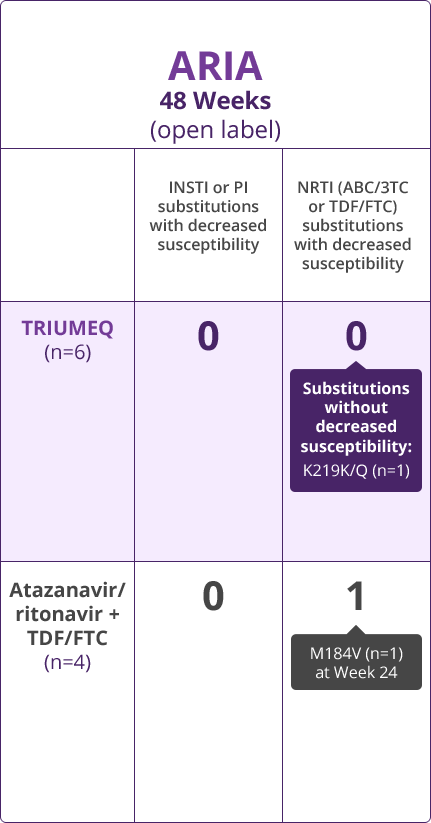
Resistance Results
- Direct comparisons across trials should not be made due to differing trial designs


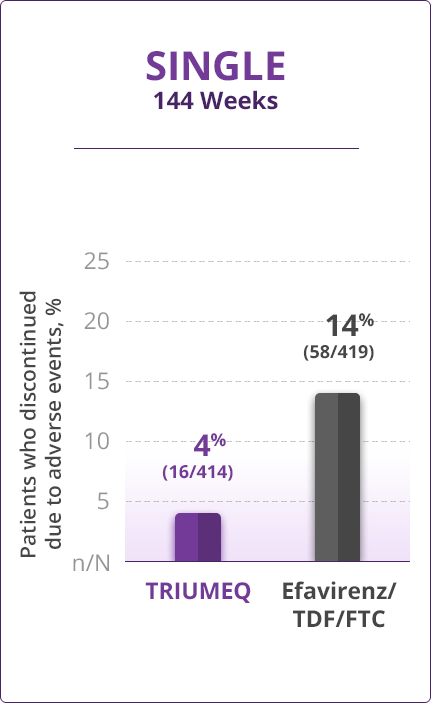
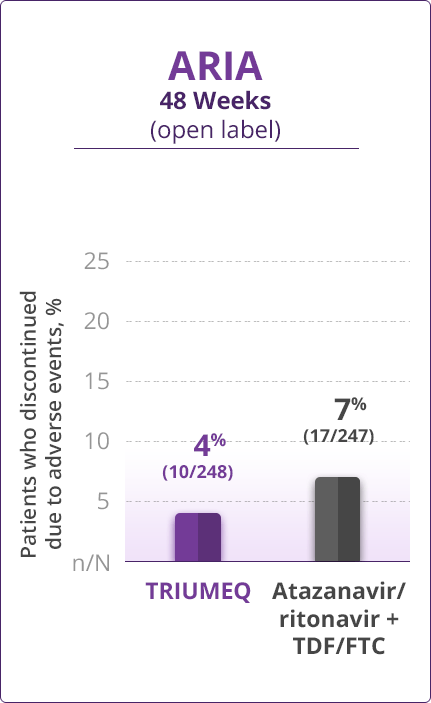
Discontinuations Due to AEs
- Direct comparisons across trials should not be made due to differing trial designs


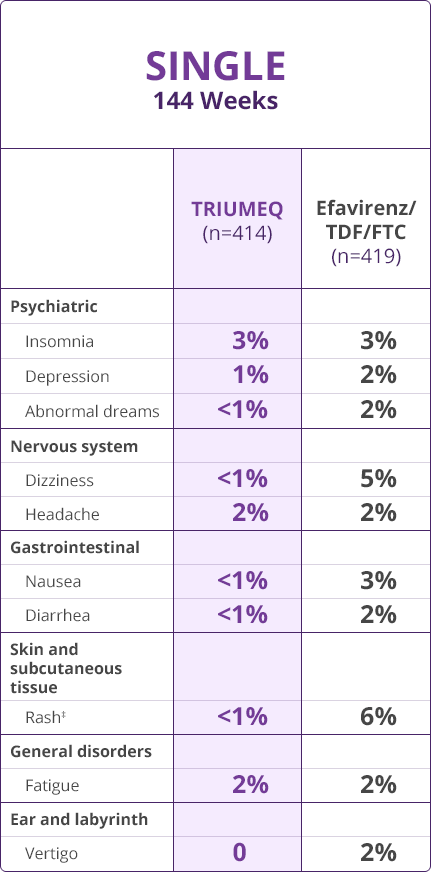
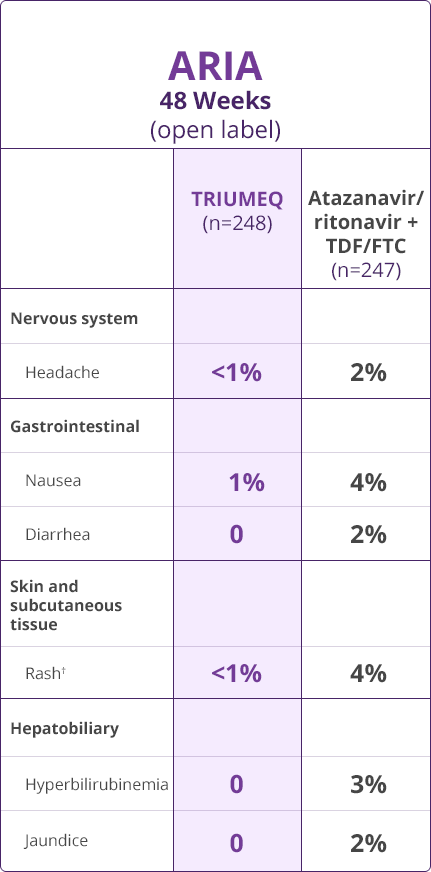
Adverse Drug Reactions (ADRs)
- Direct comparisons across trials should not be made due to differing trial designs
Please see full Prescribing Information, including Boxed Warning and Medication Guide, for TRIUMEQ and TRIUMEQ PD.
References
- Walmsley S, Baumgarten A, Berenguer J, et al. Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr. 2015;70(5):515-519.
- Orrell C, Hagins DP, Belonosova E, et al; on behalf of the ARIA Study Team. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3 study. Lancet HIV. 2017;4(12):e536-e546.
- Data on file, ViiV Healthcare.
DALWCNT220006